PARS PLANA VITRECTOMY - INTRAOPERATIVE CHALLENGES AND COMPLICATIONS SECTIONS
Visualization
Complications from air
Choroidal problems
Other complications
PARS PLANA VITRECTOMY – POSTOPERATIVE COMPLICATIONS
-
Intraocular pressure
Corneal edema
Wound leak
Hemorrhage
Gas-related complications
Oil-related complications
Other postoperative complications
SCLERAL BUCKLE - INTRAOPERATIVE CHALLENGES AND COMPLICATIONS
-
Complications during exposure
Complications from suturing or belt loops
Complications from subretinal fluid drainage
Complications from cryotherapy
SCLERAL BUCKLE – POSTOPERATIVE COMPLICATIONS
PARS PLANA VITRECTOMY -- INTRAOPERATIVE CHALLENGES AND COMPLICATIONS
Back to topVisualization
Inability to visualize the infusion canula
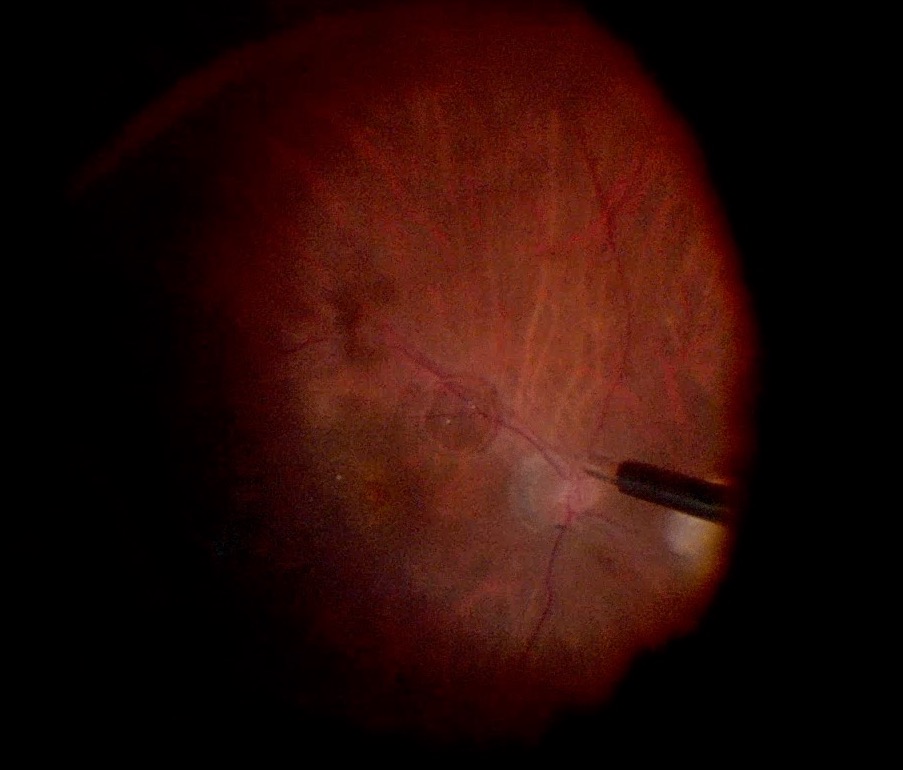
Always visually confirm the location of the infusion canula in the vitreous cavity before turning the infusion on [FIGURE 0]
Click an image to enlarge
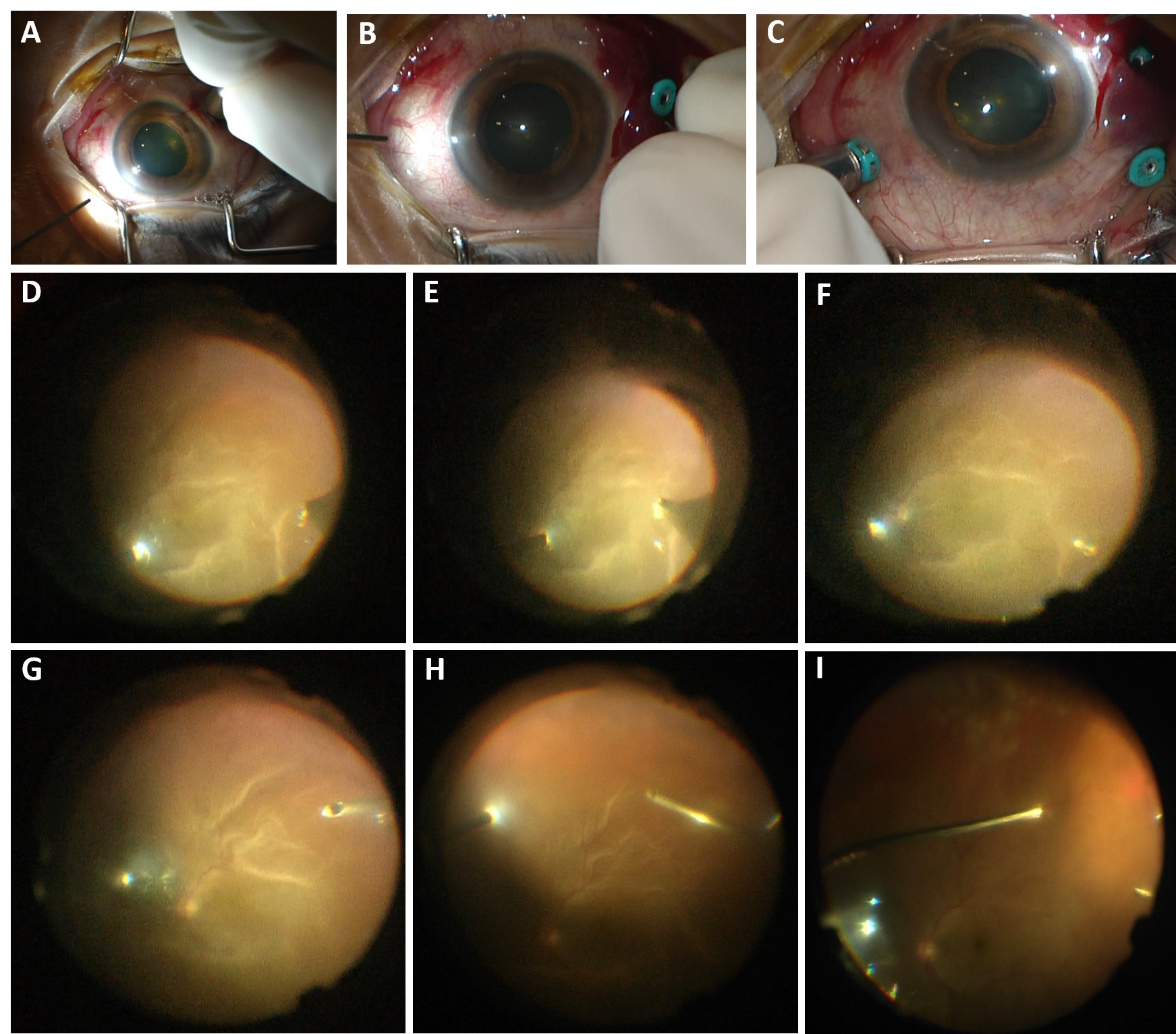
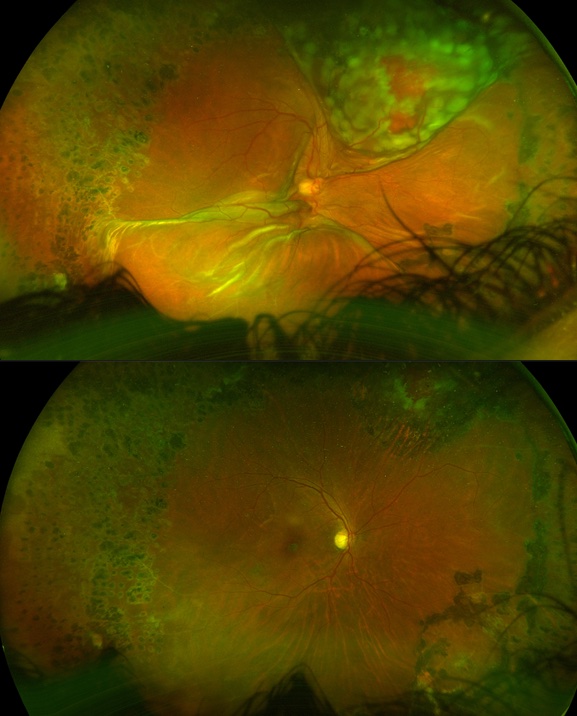
Sub-choroidal Infusion in multiple ports, with management Sub-choroidal infusion cannula in a patient with chronic macula-involving retinal detachment. The infusion was visualized to be subchoroidal in the inferotemporal (A) vitrectomy cannula and thus, it was not turned on. The infusion was moved to alternate cannulas, but noted to be sub-choroidal in the superotemporal (B) and superonasal (C) cannulas as well. A 6mm infusion tip was not available. The infusion tip was visualized to be almost through the choroid superotemporally, and thus the infusion cannula was placed superotemporally and then under wide-angle ReSight visualization, the canula was manually pushed inward toward the globe to penetrate the choroidal space (D-F). Once confirmed to be in the vitreous cavity (F), the infusion was turned on. The vitrector was inserted inferotemporally to penetrate the choroid inferotemporally and limited vitrectomy was performed (G). The infusion canula was moved inferotemporally, confirmed to be in the vitreous cavity with wide angle ReSight visualization (H), and then turned on. The case was then performed as per standard, but with frequent checking of the infusion tip to ensure no slippage sub-choroidally, including immediately prior to fluid-air exchange (I). The case was completed without incident.
Photo by Mrinali Gupta, MD
Management
- Pre-empt issues with infusion canula visualization by
- Selecting an infusion location that avoids obstacles like large choroidals
- Use a 6mm infusion canula in cases where issues are anticipated
- Address fixable issues with visualization (like miosis)
Addressing visualization issues for infusion visualization
Video by Mrinali Gupta, MD
- Move the infusion canula to a different canula site. The infusion can be moved back to the original location later in the case when visualization allows.
Moving the infusion canula to a different canula site
Video by Mrinali Gupta, MD
Place the infusion in the AC. The infusion can be moved back to the original location later in the case when visualization allows.
Using an AC infusion
Video by Mrinali Gupta, MD
Issues with the cornea
Corneal pathology noted pre-operatively that limit visualization
Management
Medical management prior to vitrectomy (e.g. IOP reduction, steroids, glycerin, etc for microcystic edema; EDTA for band keratopathy; steroids, sodium chloride topically for stromal edema)
Staged procedure after surgery to address the cornea, or (iii) combined corneal and retinal procedure with penetrating keratoplasty or temporary keratoprosthesis
PPV with temporary keratoprosthesis
Video by Mrinali Gupta, MD
When visual potential is unclear due to inability to visualize the posterior segment, endoscopy for diagnostic purposes and to assess prognosis may be considered prior to committing to corneal transplant. Endoscopic surgery may also be performed.
Endoscopy in the setting of opacified cornea
Video by Barton Blackorby, MD
Intraoperatively noted corneal problems
Epithelial edema
Risk reduction: ensuring good corneal wetting during surgery, especially in long cases like combined buckle / vitrectomy surgery
Management: epithelial debridement (e.g. #57 Beaver blade)
Corneal epithelial debridement
Video by Mrinali Gupta, MD
Leaving the limbal epithelial cells intact improves healing
Corneal endothelial edema
Risk factors: pre-existing corneal endothelial pathology, combined cases with phacoemulsification, longer surgeries, high infusion pressures intraoperatively, or marked IOP fluctuation during surgery
Management: viscoelastic application to the corneal endothelium
Viscoelastic application to the corneal endothelium
Video by Mrinali Gupta, MD
Striate keratopathy can worsen in the setting of fluid-air exchange
Management options
If mild, can be alleviated by using a noncontact viewing system or with the contact viewing system, using ample viscoelastic and minimizing posterior pressure on the cornea
Coat endothelium with viscoelastic
Viscoelastic into the AC for k edema or AC air
Video by Mrinali Gupta, MD
Return to fluid and complete the case under fluid (e.g. for retinal detachment, use PFO)
Completing the case under PFO
Video by Mrinali Gupta, MD
Issues with the anterior chamber
Remember that eyes with vitreous hemorrhage, trauma, etc may have RBCs in the anterior chamber that limit visualization. An AC washout can significant improve the view. Hemorrhage may come forward again requiring a repeat washout after the posterior segment is cleared up more.
Anterior chamber washout
Video by Mrinali Gupta, MD
Issues with the iris/dilation
Poor dilation
Risk factors: poor pre-operative dilation, history of ocular trauma, use of alpha-1a antagonists, history of uveitis/synechiae
Management:
- Stronger topical drops may be considered (phenylephrine 10% if anesthesiology feels safe to use in setting of cardiovascular status)
- Intracameral pharmacologic therapies: 1:10,000 preservative-free epinephrine
- Viscomydriasis
- Synechiolysis using viscoelastic/cannula, Kuglan hook, cyclodialysis spatula, etc.
- Mechanical devices:
- Iris hooks
Iris hooks for iris dilation.
Photo by Mrinali Gupta, MD
Iris hooks for visualization
Video by Mrinali Gupta, MD
Malyugan ring (requires larger corneal wound than typically made in vitrectomy surgeries)
Malyugen ring for iris dilation
Photo by Kyle Kovacs, MD
Surgical pupilloplasty
Issues with the natural lens
Pre-existing cataract
Management
- Vitrectomy first: if visualization and access to surgical planes (i.e. not appropriate in cases of anterior PVR or where anterior vitreous needs to be addressed) sufficient for surgical maneuvers, with the understanding that cataract will likely progress after vitrectomy and require subsequent cataract surgery
- Vitrectomy staged to be performed after cataract surgery: when pre-existing cataract limits visualization or access for the vitrectomy procedure and scheduling issues and the retinal pathology allow
- Combined cataract with intraocular lens implantation and vitrectomy
Intraoperative lens opacity limiting visualization
Causes
- Metabolic
- Increased risk with long surgical time and increased exposure to irrigating fluids and in diabetic patients
- Using irrigating solutions with dextrose, anti-oxidants, and buffers may reduce this risk
- Trauma to the lens intraoperatively
- Risk factors include: shaving of anterior vitreous, laser to anterior pathology, crossing the visual axis with instruments in phakic eyes.
- Risk can be mitigated by avoiding crossing the visual axis when working on anterior retina, using curved lasers appropriately, using scleral depression to facilitate peripheral shaving and laser
- Lens touch from instruments
- Mild, can reverse
- Lens touch with posterior capsular defect, usually from vitrector probe eating a piece of capsule/lens
Lens trauma from instruments during vitrectomy
Video by Nemo Patel, MD
- Progressive. Often worsens intraoperatively
- Consider lensectomy (phacoemulsification or pars plana lensectomy with placement of lens implant vs. plan for subsequent IOL placement (if prior lens calculations were not done). Consider leaving anterior capsule intact to allow subsequent sulcus-IOL placement)
Issues with the intraocular lens
Fogging of the IOL under air after fluid-air exchange
Condensation on the posterior IOL surface during fluid-air exchange
Photo by Mrinali Gupta, MD
Due to warm air coming in contact with the cooler IOL results in condensation – more notable in setting of prior YAG capsulotomy
Management
- Coat the posterior IOL with viscoelastic
Coating the posterior lens surface with viscoelastic
Video by Mrinali Gupta, MD
- Squeegee the condensation off with a soft-tip
Squeegee technique to remove condensation on the posterior lens surface
Video by Mrinali Gupta, MD
- Return to fluid and complete the case under fluid (e.g. for retinal detachment, use PFO to flatten the retina and complete laser, then perform PFO-air-gas exchange or PFO-gas or PFO-oil exchange)
Completing the case under PFO
Video by Mrinali Gupta, MD
- Air-silicone oil exchange (IOL fogging will go away when the oil reaches the back of the IOL), then drain subretinal fluid and complete any laser etc. under oil
Hemorrhage in Berger’s space
Occasionally, there may be hemorrhage/RBC in Berger’s space (potential space between the posterior lens capsule and anterior hyaloid)
Work through it if possible, but if view significantly compromised, create an opening in the anterior hyaloid to facilitate release of the hemorrhage
Removing hemorrhage in Berger's space
Video by Mrinali Gupta, MD
Problems with hemostasis
Sites of bleeding
Scleral
at sclerotomy site
Ciliary body
e.g. too anteriorly placed sclerotomies, removal of cyclitic membrane, etc
Iris
during inferior iridotomy, neovascularization of the iris
Retina
from neovascular membranes or from retinal vessels due to breaks, retinotomy/retinectomy, iatrogenic breaks
Choroid
Maneuvers that may cause bleeding
Dissection of neovascular membranes, membrane peeling, PVD induction, retinectomy/retinotomy, chorioretinal biopsy, iatrogenic retinal strikes, etc.
Management of hemostasis
Pre-operative risk management
- Addressing the systemic factors contributing to bleeding
- Evaluation (blood count, coagulation studies, etc) and management, if possible, of bleeding/coagulation disorders
- Modification of antiplatelet or anticoagulants, if possible, based upon discussion of risks vs. benefits with primary care physician / internist
- Addressing ocular factors
- Timing of surgery (e.g. allowing vascular congestion to be reduced after trauma, inflammatory conditions, retinopathy of prematurity, etc, before operating)
- Pre-operative laser (e.g. in proliferative diabetic retinopathy)
- Pre-operative anti-vascular endothelial growth factor therapy (e.g. in proliferative diabetic retinopathy). Note risk of “crunch” if fibrovascular membranes contract rapidly, especially in patients where compliance with followup/surgeries is a concerns
- Intraoperative risk management
- Addition of preservative-free epinephrine (0.1-0.3cc of 1:1000 to a 500 cc bottle of irrigation fluid) to irrigating fluids
- Use of balanced salt solution without citric acid component (an anticoagulant) for irrigating solution
- Applying diathermy prior to retinotomy or retinectomy
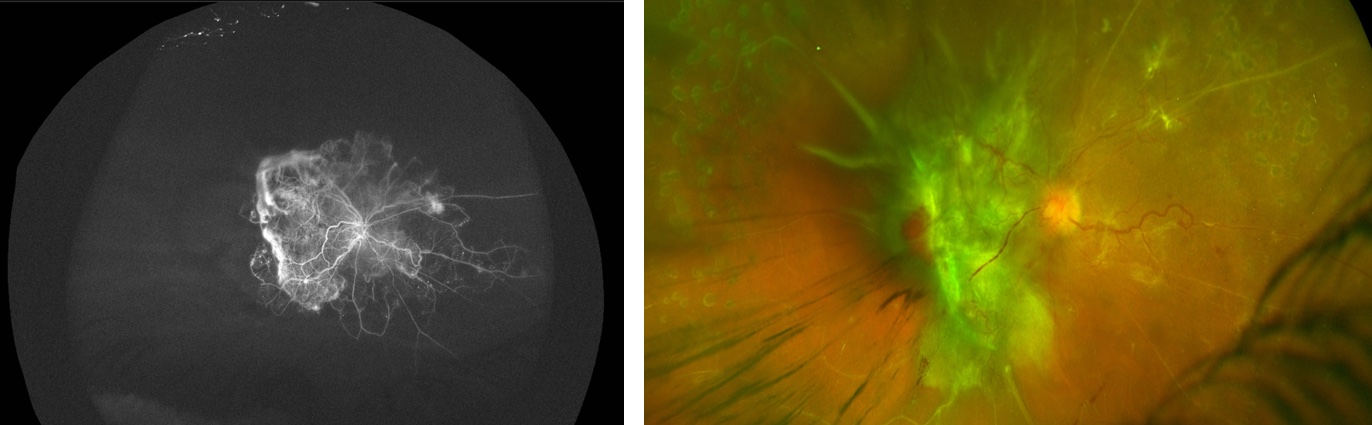
Pre-op anti-vegf and laser prior to TRD repair
Photo by Mrinali Gupta, MD
Diathermy for retinectomy
Photo by Mrinali Gupta, MD
Managing intraocular hemorrhage intraoperatively
- Increase infusion pressure: avoid increasing pressure above arterial pressure (arterial pulsations will be seen at this point) to prevent retinal artery occlusion/ischemia
- Fluid air exchange: multiple desired effects including: (1) hemorrhage concentrates in the fluid component, allowing the air-filled area to be clear and improve visualization (2) higher surface tension of gas promosed hemostasis, and (3) potentially concentrates the coagulation factors in a smaller (fluid) area over the retina to promote coagulation
- Heat: laser or diathermy at the site of bleeding if possible
Applying Diathermy to the source of the intraoperative hemorrhage
Video by Kyle Kovacs, MD
- Direct pressure: mechanical pressure applied gently with a blunt tipped instrument such as the vitrector, soft-tip cannula, or specialized instruments for this purpose
Direct pressure with an instrument to control intraoperative hemorrhage
Video by Patrick Starapoli, MD + Kenneth Fan, MD
- Viscoelastic: application to the site of hemorrhage may provide a tamponade effect to promote thrombosis
- Perfluorocarbon: Application may provide a tamponade effect to promote hemostasis
- Silicone oil: may provide an internal tamponade and compartmentalize the hemorrhage. However, in oil-fill eyes, there may be compartmentalization of pro-inflammatory factors in the fluid meniscus that increase risk of proliferative vitreoretinopathy postoperatively
Complications from air
Fogging of the IOL after fluid-air exchange
Lens trauma from instruments during vitrectomy
Video by Nemo Patel, MD

Condensation on the posterior IOL surface during fluid-air exchange
Photo by Mrinali Gupta, MD
Due to warm air coming in contact with the cooler IOL results in condensation – more notable in setting of prior YAG capsulotomy
Management
- Coat the posterior IOL with viscoelastic
Coating the posterior lens surface with viscoelastic
Video by Mrinali Gupta, MD
- Squeegee the condensation off with a soft-tip
Squeegee technique to remove condensation on the posterior lens surface
Video by Mrinali Gupta, MD
- Return to fluid and complete the case under fluid (e.g. for retinal detachment, use PFO to flatten the retina and complete laser, then perform PFO-air-gas exchange or PFO-gas or PFO-oil exchange
Completing the case under PFO
Video by Mrinali Gupta, MD
- Air-silicone oil exchange (IOL fogging will go away when the oil reaches the back of the IOL), then drain subretinal fluid and complete any laser etc. under oil
Worsening striate keratopathy (corneal endothelial edema) under air
Management
- If mild, can be alleviated by using a noncontact viewing system or with the contact viewing system, using ample viscoelastic and minimizing posterior pressure on the cornea
- Coat endothelium with viscoelastic
Viscoelastic application to the corneal endothelium
Video by Mrinali Gupta, MD
- Return to fluid and complete the case under fluid (e.g. for retinal detachment, use PFO to flatten the retina and complete later, then perform PFO-air-gas exchange or PFO-gas or PFO-oil exchange
Completing the case under PFO
Video by Mrinali Gupta, MD
Air migration into the anterior chamber in aphakic eyes or eyes with zonular defect
Compromises visualization
Management
- Fill the anterior chamber with viscoelastic to act as a barrier to air entry
- Consider placing a vent in one of the cannulas as a conduit for air release (such that air will vent through it, rather than coming anteriorly). Either add another port or if only laser is required, perform laser using a single port with a lighted laser probe
- Return to fluid and complete the case under fluid (e.g. for retinal detachment, use PFO to flatten the retina and complete laser, then perform PFO-air-gas exchange or PFO-gas or PFO-oil exchange.
Completing the case under PFO
Video by Mrinali Gupta, MD
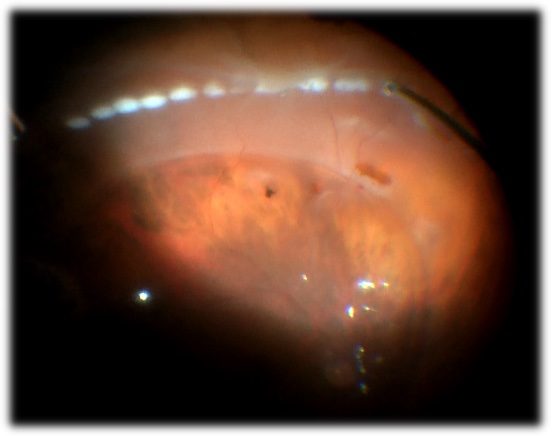
Subretinal air
Subretinal air during vitrectomy
Video by Nemo Patel, MD
Subretinal air
Photo by Nemo Patel, MD
Clamp the infusion
Confirm that the infusion canula is in the vitreous cavity
- If not, stop the infusion, move the infusion to a different location
- If the infusion canula is correctly positioned, subretinal air is a sign that sufficient traction was not relieved prior to fluid-air exchange. Return to fluid, relieve traction and remove the subretinal air prior to returning to air
Complications related to PFO
Retained PFO
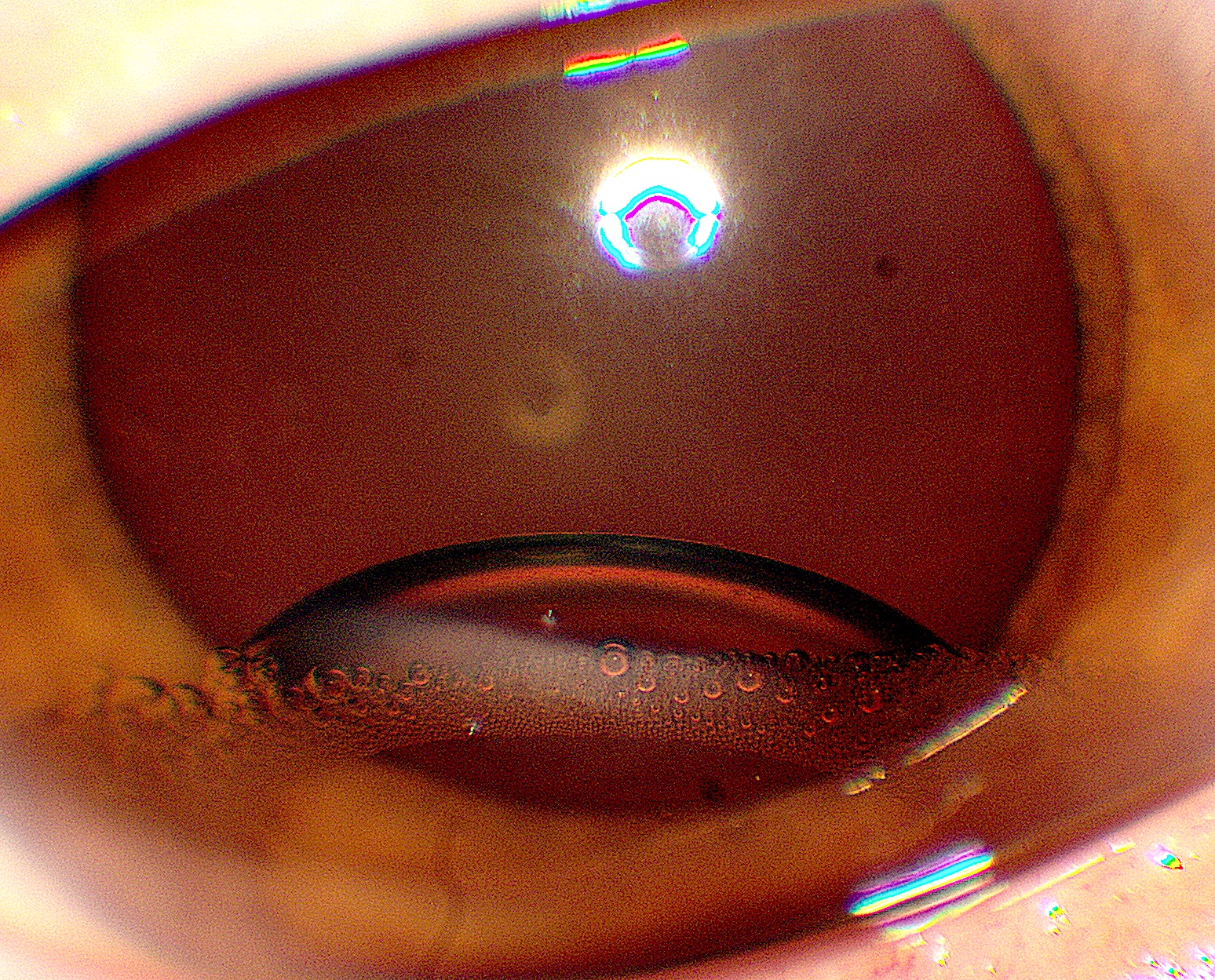
Retained PFO in the anterior chamber
Photo by Donald J. D’Amico, MD
Retained PFO in the posterior chamber
Minimize risk by avoiding bubbles of PFO during infusion by
1) Keeping the PFO canula within the bubble of PFO at all times but sufficiently retracted so that the fluid egress port of the dual bore canula itself is not in the PFO
2) Avoid PFO overfill
3) Complete removal of all of the PFO under air
To ensure removal during surgery, may wait a few minutes to allow retained PFO and fluid to re-accumulate and remove the PFO a second time under air to ensure removal
To ensure removal during surgery, may apply a BSS rinse to the posterior pole while under air and then complete the fluid-air exchange
BSS rinse for PFO removal
Video by Thanos Papakostas, MD
To ensure removal during surgery, can open one sclerotomy (remove valve or unplug) to allow air to flow through the eye while under air. PFO is very volatile and small amounts will evaporate.
Subretinal PFO
Subretinal PFO
May cause retinal and retinal pigment epithelial toxicity with secondary scotomy; may predispose to inflammation
Risk mitigation
- Avoid a forceful stream of PFO during infusion
- Avoid aiming at the macula/fovea or retinal breaks
- Avoid PFO bubbles during PFO infusion (keep the tip of the dual bore canula in the bubble but keep the egress port in fluid component and out of PFO)
- Position the eye appropriately when injecting PFO in cases of large breaks or retinectomies
Can also intraoperatively during use of PFO use with persistent traction on the retina, allowing the PFO to get under the retina. Manage by draining the subretinal PFO and relieving traction before proceeding further
Complications from silicone oil
Migration into the anterior chamber during infusion
Intraoperative anterior chamber migration of silicone oil
Photo by Kyle Kovacs, MD
Risk factors: silicone oil overfill, aphakia
Management
- Insertion of viscoelastic into the anterior chamber to displace the oil posteriorly or out through the paracentesis
Viscoelastic application into the anterior chamber for intraoperative oil migration
Video by Kyle Kovacs MD + Mahmood Khan MD
- Any residual anterior chamber silicone oil can be removed from the anterior chamber using
- Viscoelastic extrusion cannula inserted through a paracentesis incision
- Large bore butterfly needle attached to a syringe with manual exertion of negative pressure
- For smaller bubbles, passive effusion through posterior pressure on the paracentesis while injecting viscoelastic into the anterior chamber as above)
Finally, remember to titrate the posterior segment silicone oil fill (removed if overfill added if additional fill necessary
Iatrogenic breaks
Causes: thin or ischemic retina, traction during PVD induction or membrane peeling/dissection, strike with surgical instruments, vitreoretinal traction due to maneuvering of instruments in the eye (especially near sclerotomy sites), fluid dynamics causing vitreoretinal traction (relieved by using low vacuum high cut rate settings during vitreous shaving)
Scleral depression to examine the retina thoroughly at the end of every case and identify/treat breaks to reduce post-op detachments/re-detachments
Management
- Ensure relief of all traction from the break – including vitreous and membrane-related traction. Apply laser or cryotherapy. If there is associated subretinal fluid, fluid-air exchange may be required to drain the fluid prior to laser. Consider tamponade with air or gas, especially if associated subretinal fluid was noted.
- If all traction cannot be removed, consider a focal retinectomy
Iatrogenic retinal break
Video by Mrinali Gupta, MD
- Posterior breaks near the nerve or fovea often do not require laser, but all traction should be removed from the break
Choroidal problems
Choroidal effusion
Intraoperative choroidal effusion
Video by Kyle Kovacs MD
Due to imbalance between infusion in and extrusion out of the eye.
For example if the infusion is blocked, kinked, or coming out; if the infusion pressure is low relative to the vacuum setting being used or if hybrid cases are being done and the infusion canula gauge is smaller than the surgical instrument guage
Management is centered around finding the problem and fixing it:
- Increase the infusion pressure
- Always consider the possibility of a suprachoroidal hemorrhage (dark underlying color, blood noted, no resolution upon elevation of intraocular pressure) and manage appropriately
- If the effusion does not resolve at high pressure and a suprachoroidal hemorrhage is not suspected, confirm that the infusion has not slipped out / is in the vitreous cavity.
- If the infusion is in place but the effusion remains, check for kinks in the infusion line. Consider that vitreous or large/dense hemorrhage may be blocking the inside port of the infusion canula. If so, re-position the infusion to another canula site or the AC until this is evacuated. The infusion can then be returned to its original location.
Suprachoroidal hemorrhage
Risk factors
- Older patients, hypertension or tachycardia, anticoagulation, history of glaucoma (especially with pre-operative IOP elevation), high myopia, aphakia, pseudophakia, coughing/bucking, external drainage of subretinal fluid
Due to sudden decrease in IOP (especially if elevated previously) and in the setting of high systemic blood pressure, which causing rupture of short or long posterior arteries, resulting in hemorrhage. If there is forced separation of the choroid from the sclera, then the vessels will be further avulsed, with further bleeding.
- Elevated choroidal often with dark underlying color
Pre-operative risk mitigation
- Modulate anticoagulation if possible based on discussion of risk vs. benefits with primary care provider, optimization of heart rate and blood pressure parameters preoperatively, pre-operative IOP control
Intraooperative management
- The key is to stabilize the IOP
- Elevate infusion pressure
- Especially useful if unclear if choroidal is hemorrhagic versus serous intraoperatively. The latter will usually improve with IOP increase
- Make sure the infusion canula has not slipped into the choroidal space
- For small localized suprachoroidal hemorrhage and urgent/emergent surgery (retinal detachment), surgery may be continued if it remains small and localized. For larger or progressive suprachoroidal hemorrhage, immediately stop surgery and close all wounds
- Small choroidal hemorrhages often resolve. Large hemorrhages or increasing hemorrhages resulting in kissing choroidals or other complications may require subsequent drainage, ideally 10 – 20 days postoperatively to allow time for the eye to quiet down / stabilize and for clot lysis
- Techniques for drainage
- Cut down with blade
Choroidal drainage technique: scleral cut down
Video by Thomas Lazzarini MD + Harry Flynn MD
- Use of the cannula (valve removed)
- Use of PFO for internal pressure to facilitate drainage
Poor visual prognosis
Other complications
Retinal incarceration in the canula
Moving the infusion canula to a different canula site
Video by Mrinali Gupta, MD
PARS PLANA VITRECTOMY – POSTOPERATIVE COMPLICATIONS
Back to topIntraocular pressure
Elevated intraocular pressure
Open angle
- Post-operative/inflammatory (risk factors: prolonged surgery, multiple surgeries, intraoperative lensectomy, extensive endolaser, preexisting uveitis), steroid response, retained viscoelastic, gas overfill (without pupillary block), oil overfill (without pupillary block), other causes of secondary open angle glaucoma (hyphema, ghost cell, etc)
- Management generally involves topical or oral therapy for medical management of IOP, steroid drops for inflammatory component or steroid taper for steroid-response component, and addressing the underlying etiology. Anterior chamber paracentesis may be required for immediate pressure reduction in the event that medical therapy is unsuccessful or for removal of retained viscoelastic material
Angle closure
- Gas overfill (with pupillary block), oil overfill (with pupillary block), appropriate oil fill in an aphakic eye or in a pseudophakic eye with zonular instability without patent inferior iridotomy, synechial angle closure, aqueous misdirection, choroidal effusion, choroidal hemorrhage
- Management: step-wise until IOP control achieved:
- Topical or systemic IOP-lowering medication. Anterior chamber paracentesis may be required for immediate pressure reduction in the event that medical therapy is unsuccessful
- If the above fails and due to pupillary block component, laser peripheral iridotomy. Also perform an iridotomy to rule out pupillary block component in suspected cases of aqueous misdirection. Vitrectomy to address the hyaloid face (if still intact) may be required if aqueous misdirection is suspected and neither medical control nor iridotomy achieve IOP control.
- If the above fails and due to gas or oil overfill, face down positioning or removal of some gas or oil (in-office exchange or return to the operating room)
- If the above fails and due to choroidal effusion and/or hemorrhage, consider drainage. *(Link to choroidal complication section)*
- If the above fails and complete synechial angle closure noted, referral to glaucoma specialist for consideration of a tube shunt
Low intraocular pressure
See: wound leakCorneal edema
Risk factors: underlying corneal disease, long duration of surgery, elevated intraocular pressures or significant pressure fluctuations during surgery, significant IOP elevation or low IOP postoperatively, debridement of the corneal epithelium during vitrectomy surgery
Management: topical steroids, IOP control
Wound leak
Clinical features: low IOP, Seidel positively of wounds, significant fluid or air/gas bleb under the conjunctiva near a sclerotomy, gas or oil underfill, secondary hyphema/vitreous hemorrhage, secondary choroidal effusions and/or subsequent choroidal hemorrhage
Risk factors: larger wounds/smaller gauge sclerotomies, sutureless surgery, use of fragmatome with secondary heating of the sclera, lack of beveled incision, fluid-filled eye (vs. gas or air-filled, oil filled), scleral wounds associated with sutured intraocular lens placement
Management
Small corneal wound leaks
Bandage contact lens, aqueous suppressants and observation
If conservative measures fail, wound revision/suturing
Large corneal wound leaks
Glue or wound revision/suturing. Donor/dehydrated corneal graft or scleral patch graft may be required in the setting of tissue loss or weak tissue
Scleral wound
Close monitoring for small scleral wound leaks, which typically resolve
Large scleral wound (e.g. 20g fragmatome sites, sites of foreign body removal) leaks or scleral wound leaks that were monitored without significant improvement and/or worsening of sequelae (choroidals, etc), warrant revision of the wound in the operating room
Hemorrhage
Hyphema
Management: topical steroids, cycloplegia, topical and/or oral IOP lowering medications. If medical management is not successful, anterior chamber washout
Vitreous hemorrhage
Management: observation, assessment for cause of the vitreous hemorrhage (e.g. ?new retinal tear) and B-scan ultrasonography for monitoring of the posterior segment while awaiting clearance, return to the operating room for non-clearing vitreous hemorrhage or IOP elevation due to vitreous hemorrhage/ghost cell glaucoma
Cataract
Nuclear sclerosis cataract
Management: non-urgent cataract extraction
Post-vitrectomy lens feathering
- Common after vitrectomy, especially in the setting of postoperative lens-gas touch.
- Management: likely to resolve in time, especially with adequate face-down positioning/gas resorption
Iatrogenic cataract from lens touch
Risk factors include: shaving of anterior vitreous, laser to anterior pathology, crossing the visual axis with instruments in phakic eyes.
Risk can be mitigated by avoiding crossing the visual axis when working on anterior retina, using curved lasers appropriately, using scleral depression to facilitate peripheral shaving and laser
- Lens touch from instruments
Lens touch from a vitrectomy instrument
Photo by Kyle Kovacs, MD
- Mild, can reverse
- Lens touch with posterior capsular defect
- Usually from vitrector probe eating a piece of capsule/lens
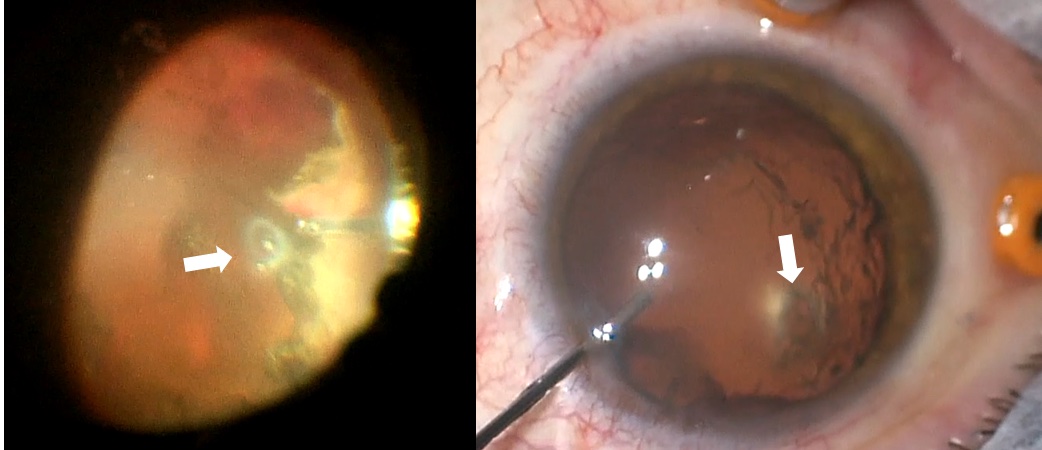
Lens touch during vitrectomy with a posterior capsular defect
Photo by Nemo Patel, MD + Frances Wu, MD
Iris hooks for visualization
Video by Mrinali Gupta, MD
- Progressive, often rapidly progressive
- Management
- Schedule lensectomy (or cataract extraction with retina specialist available in the setting of high risk for dropped nucleus). Timing of surgery depends on clinical course and other posterior segment pathology (e.g. if noted post RD-repair, monitor closely and attempt to address the cataract several weeks or more after RD repair to allow retinal reattachment before reoperating). While awaiting surgery, monitor closely for inflammation and IOP elevation which may warrant medical management or sooner return to the operating room. Phacoanaphylactic glaucoma may occur.
Gas-related complications
Gas underfill
Results in insufficient tamponade, especially of inferior retinal pathology
Risk factors: incomplete drainage of subretinal fluid, presence of choroidal effusions or hemorrhage during fluid-air exchange prior to air-gas exchange, inaccurate gas concentration calculation, leakage from sclerotomies
Management
- Attempt to achieve tamponade of the retinal pathology with strict prone positioning
- If the gas bubble is much too small to tamponade the pathology even with strict positioning, consider increasing gas fill via intravitreal injection of a small volume of expansile gas or via a fluid-gas exchange
Gas overfill
Clinical features include high IOP, either open angle or closed angle-closure due to pupillary block
Risk factors: inaccurate gas concentration calculation (usage of expansile gas rather than isoexpansile concentration), appropriate gas concentration but inappropriately high volume of gas
Management
- Control IOP via medical management
- If medical management cannot sufficiently achieved sustained IOP control, consider vitreous tap of a small amount of gas. The bulb of the syringe must be kept in place during tap to ensure controlled release of the gas
- If medical management coupled with vitreous tap do not achieved sustained IOP control, return to the operating room to remove and replace the gas.
Oil-related complications
Oil underfill
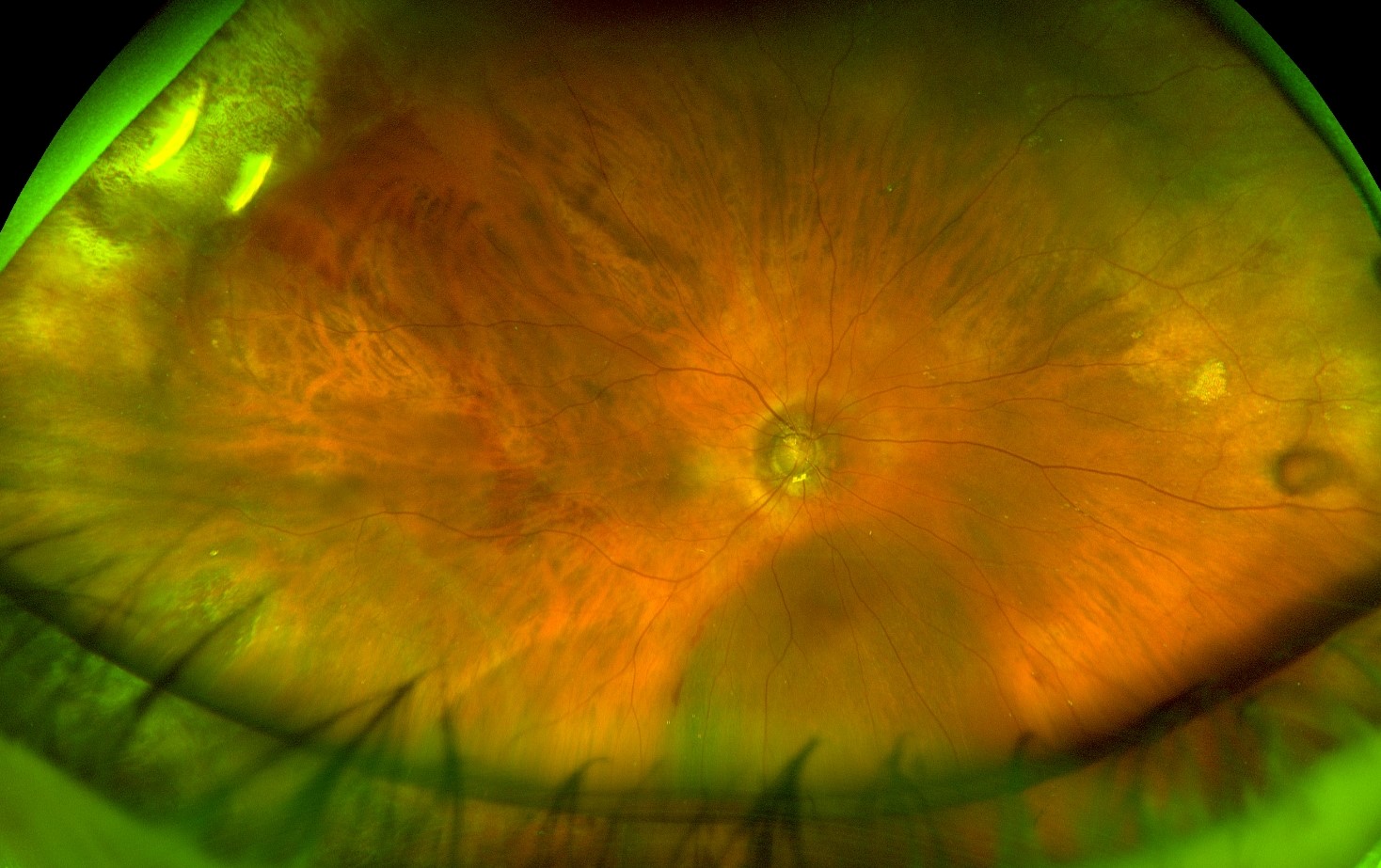
Silicone oil underfill after vitrectomy
Photo by Kyle Kovacs, MD
Results in insufficient tamponade, especially of inferior retinal pathology. Inflammatory cytokines may also collect in the large inferior fluid meniscus predisposing to proliferative vitreoretinopathy. Underfilled eyes also at higher risk of emulsification due to sheer forces in the oil with eye movement
Risk factors: incomplete drainage of subretinal fluid, presence of choroidal effusions or hemorrhage during fluid-air exchange prior to the air-oil exchange, leakage from sclerotomies
Techniques to avoid underfill
- Ensure complete drainage of fluid and subretinal fluid during fluid-air exchange, prior to air-oil exchange
- During air-gas exchange, ensure adequate venting and gradually decrease the air infusion pressure. Consider moving the vent to another port to ensure removal of trapped air pockets
- Monitor the air-oil meniscus closely. In aphakic eyes, a 30g needle may be inserted to identify the location of the glistening interface between air and oil
Management
- Attempt to achieve tamponade of the retinal pathology with strict prone positioning
Oil overfill
Clinical features include high IOP, either open angle or closed angle-closure due to pupillary block. Oil may migrate into the anterior chamber
Techniques to avoid oil overfill
- Slow air-oil exchange with careful attention to the position of the infusion line. Lower the air infusion pressure and monitor closely for oil entry into the infusion line to signal appropriate fill. Titrate the oil fill to appropriate palpated ocular pressure – do not leave the operating room with a hard eye.
- Monitor the air-oil meniscus closely. In aphakic eyes, a 30g needle may be inserted to identify the location of the glistening interface between air and oil
Using a 30g needle to assess for the air-oil interface during vitrectomy
Video by Kyle Kovacs, MD
Management
- Control IOP via medical management
- If medical management cannot sufficiently achieved sustained IOP control, consider vitreous tap of a small amount of oil. Alternatively, return to the OR to remove some silicone oil in a more controlled fashion
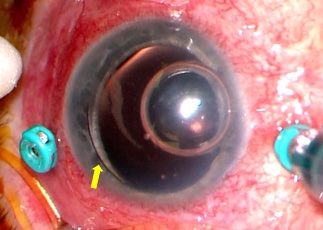
Anterior chamber silicone oil
Oil bubble may be visualized in the superior anterior chamber. IOP elevation, inflammation, and/or corneal edema may ensue
Risk factors: aphakia (especially with absent inferior iridotomy), oil overfill
Techniques to prevent anterior chamber silicone oil
- If performing lensectomy consider leaving anterior capsule with small central capsulotomy
- In aphakic eyes
- perform an inferior iridotomy
- while under air, consider instilling viscoelastic into the anterior chamber as a barrier to silicone oil
- monitor air-oil exchange closely for meniscus to avoid overfill
- if aniridic, consider oil retention sutures
- prone positioning
How to manage oil migration into the anterior chamber if noted intraoperatively
- Insertion of viscoelastic into the anterior chamber through a paracentesis to create a barrier to further anterior silicone oil migration. The silicone oil will often egress from the paracentesis during viscoelastic placement or will be displaced posteriorly
Viscoelastic application into the anterior chamber for intraoperative oil migration
Video by Kyle Kovacs MD + Mahmood Khan MD
- Any residual anterior chamber silicone oil can be removed from the anterior chamber using
- Viscoelastic extrusion cannula inserted through a paracentesis incision
- Large bore butterfly needle attached to a syringe with manual exertion of negative pressure
- For smaller bubbles, passive effusion through posterior pressure on the paracentesis while injecting viscoelastic into the anterior chamber as above
Iatrogenic retinal break
Video by Mrinali Gupta, MD
Finally, remember to titrate the posterior segment silicone oil fill (removed if overfill added if additional fill necessary
Postoperative management of anterior chamber silicone oil
- Attempt prone positioning to reposit oil posteriorly, especially if aphakic
- Consider inferior iridotomy if absent
- If significant oil bubble results in IOP elevation that is not well controlled with medical management, return to the operating room for removal.
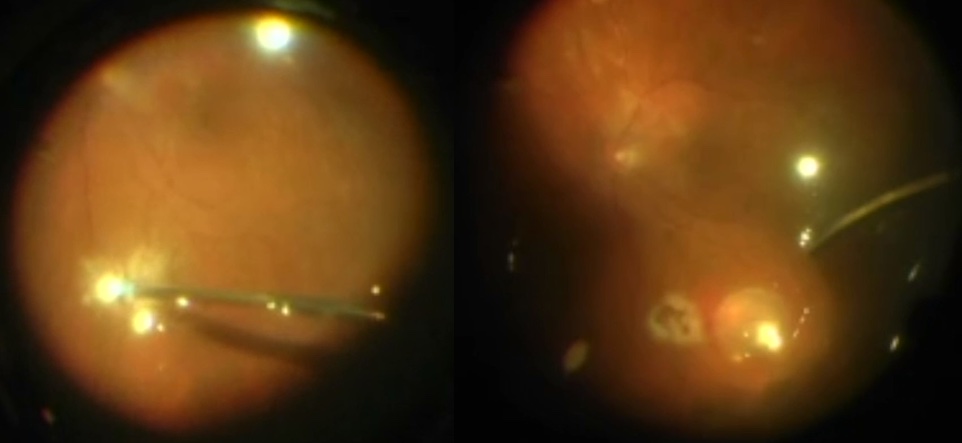
Silicone oil emulsification
Risk mitigation: avoid oil underfill, remove oil in a timely fashion
Clinical features: tiny drops of emulsified oil in the anterior chamber or vitreous, possibly inflammation and/or IOP elevation
Emulsified oil in the anterior chamber
Photo by Sabin Dang, MD + Maxwell Wingelaar, MD
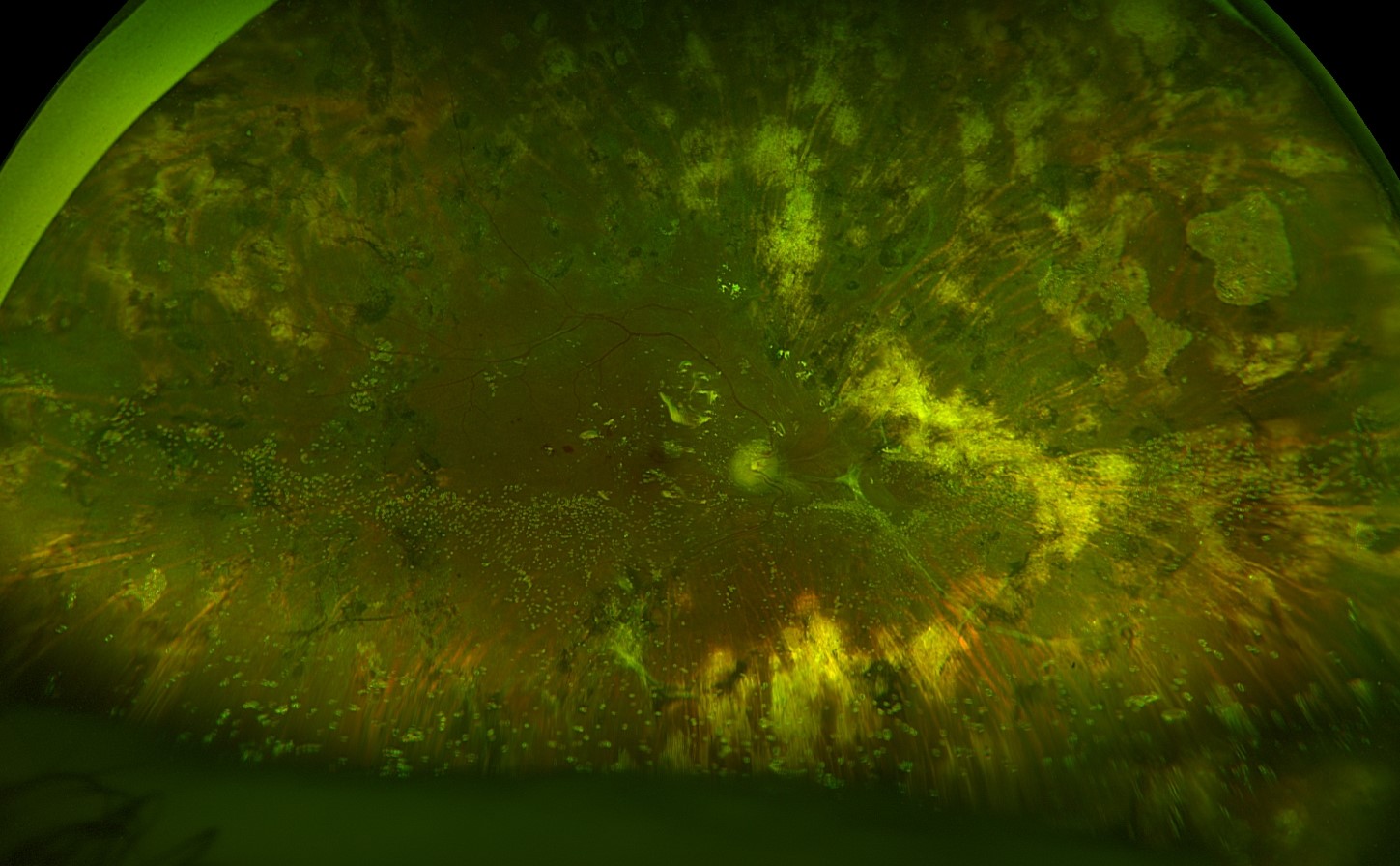
Emulsified oil in the posterior segment
Photo by Anton Orlin, MD
Manage with surgery to remove oil and emulsified oil droplets. Perform fluid-air exchange and skim oil droplets that accumulate at the interface of the fluid and air to facilitate more complete removal. Gently shake the globe to release trapped emulsified oil droplets into the posterior segment.
Using fluid-air exchange to facilitate removal of emulsified oil
Video by Kyle Kovacs MD
Complications related to PFO
Retained PFO

Retained PFO in the anterior chamber
Photo by Donald J. D’Amico, MD

Retained PFO in the posterior chamber
Minimize risk by avoiding bubbles of PFO during infusion by
1) Keeping the PFO canula within the bubble of PFO at all times but sufficiently retracted so that the fluid egress port of the dual bore canula itself is not in the PFO
2) Avoid PFO overfill
3) Complete removal of all of the PFO under air
To ensure removal during surgery, may wait a few minutes to allow retained PFO and fluid to re-accumulate and remove the PFO a second time under air to ensure removal
To ensure removal during surgery, may apply a BSS rinse to the posterior pole while under air and then complete the fluid-air exchange
BSS rinse for PFO removal
Video by Thanos Papakostas, MD
To ensure removal during surgery, can open one sclerotomy (remove valve or unplug) to allow air to flow through the eye while under air. PFO is very volatile and small amounts will evaporate.
Management
- Small retained PFO bubbles without significant sequelae or symptoms may be removed vs. observed
- Larger retained PFO bubbles or those causing significant sequelae (IOP elevation, inflammation, significant symptoms, etc) should be removed by returning to the OR. Fluid air exchange can facilitate complete removal of the PFO.
Subretinal PFO

Subretinal PFO
May cause retinal and retinal pigment epithelial toxicity with secondary scotomy; may predispose to inflammation
Risk mitigation
- Avoid a forceful stream of PFO during infusion
- Avoid aiming at the macula/fovea or retinal breaks
- Avoid PFO bubbles during PFO infusion (keep the tip of the dual bore canula in the bubble but keep the egress port in fluid component and out of PFO)
- Position the eye appropriately when injecting PFO in cases of large breaks or retinectomies
Can also intraoperatively during use of PFO use with persistent traction on the retina, allowing the PFO to get under the retina. Manage by draining the subretinal PFO and relieving traction before proceeding further
Management
- Small asymptomatic subretinal PFO bubbles may be observed, especially if inferior
- Larger subretinal PFO bubbles, subfoveal PFO bubbles, subretinal PFO bubbles superior to the fovea in the posterior pole with concern for migration into the fovea, and/or subretinal PFO bubbles causing significant visual scotoma should be considered for removal.
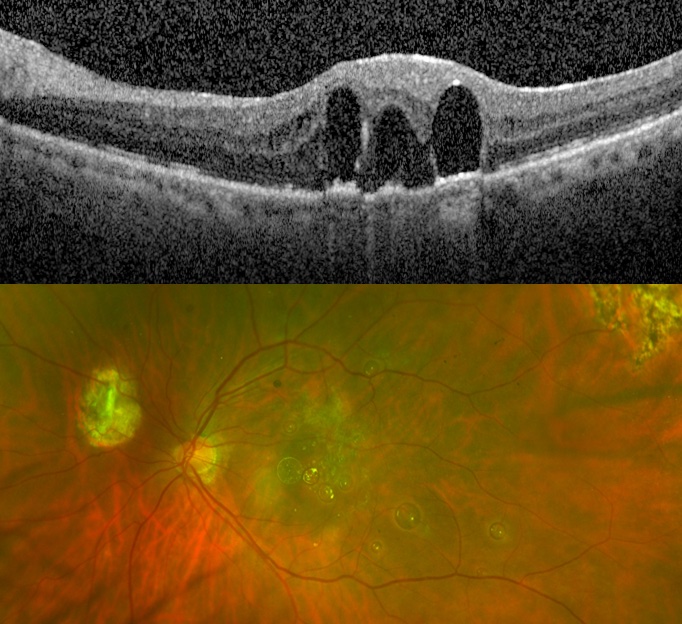
Subretinal PFO necessitating removal
Surgery for removal of subretinal PFO
Video by Donald D'Amico, MD + Kyle Kovacs, MD
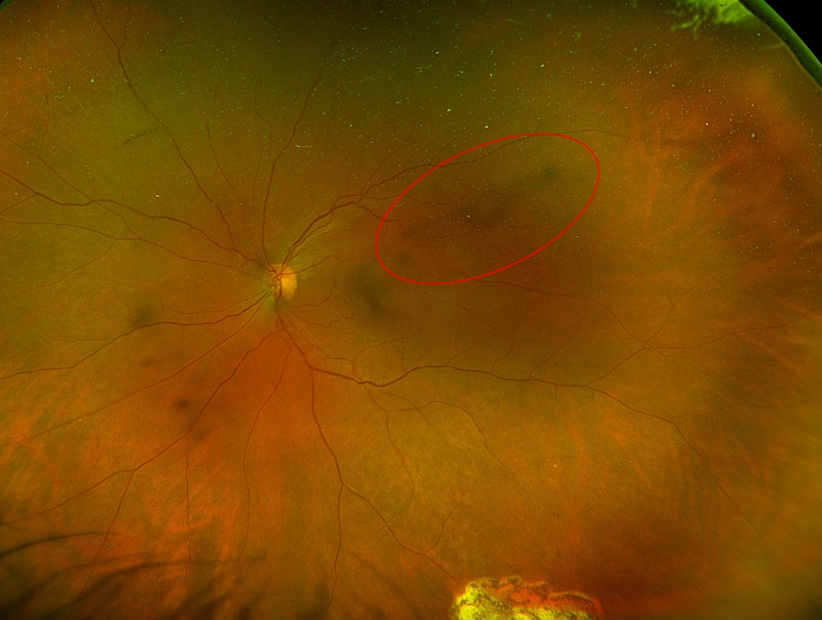
Exudative retinal detachment
Usually starts to occur several days postoperatively, color more turbid than rhegmatogenous fluid, usually inferior; fluid shifts based on head position
Risk factors include longer surgery, multiple surgeries, significant endolaser
Resolve over time. Consider topical and systemic steroids.
Choroidal complications
Choroidal effusion
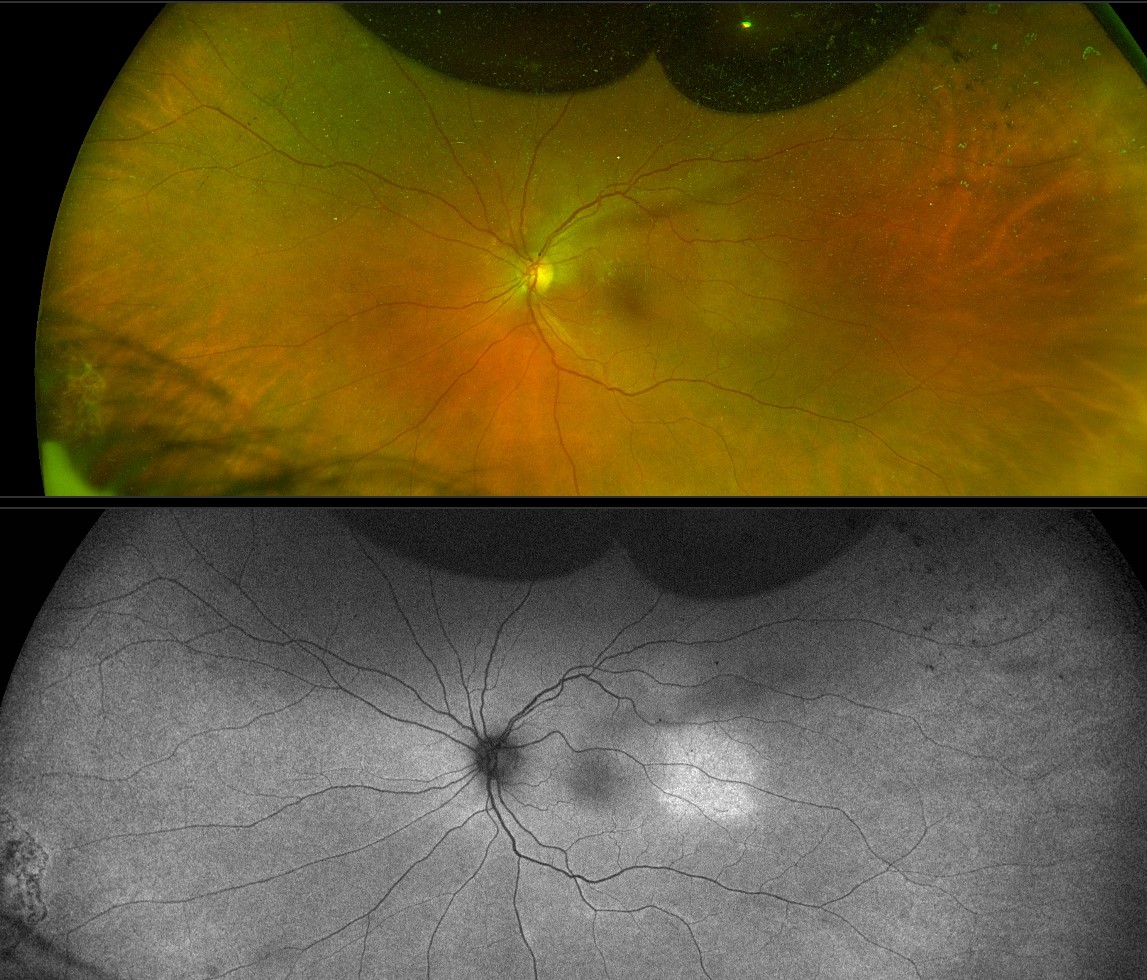
Postoperative choroidal effusion
Due to significant inflammation or hypotony.
Risk factors: wound leak/hypotony, long surgery, multiple surgeries or concomitant buckle surgery, significant endolaser application, significant inflammation or history of uveitis, smaller eyes
IOP may be elevated due to forward shift of the lens-iris diaphragm
Management:
- Topical and systemic steroids
- Atropine
- Topical or systemic IOP-lowering medications as needed
- If persistent and severe IOP elevation despite the regimen above or retinal apposition, choroidal drainage -- with anterior chamber reformation if needed
- Techniques for drainage
- Cut down with blade
Choroidal drainage technique: scleral cut down
Video by Thomas Lazzarini MD + Harry Flynn MD
- Use of the cannula (valve removed)
Suprachoroidal hemorrhage
Risk factors: Older patients, hypertension or tachycardia, anticoagulation, history of glaucoma (especially with pre-operative IOP elevation), high myopia, aphakia, pseudophakia, coughing/bucking, concomitant buckle surgery
Management
- Small choroidal hemorrhages often resolve and can be managed medically with topical and systemic steroids, atropine, and medications for IOP lowering as needed. Large hemorrhages or increasing hemorrhages resulting in kissing choroidals or other complications may require subsequent drainage, ideally 10 – 20 days postoperatively to allow time for the eye to quiet down / stabilize and for clot lysis
- Techniques for drainage
- Cut down with blade
Choroidal drainage technique: scleral cut down
Video by Thomas Lazzarini MD + Harry Flynn MD
- Use of the cannula (valve removed)
- Use of PFO for internal pressure to facilitate drainage
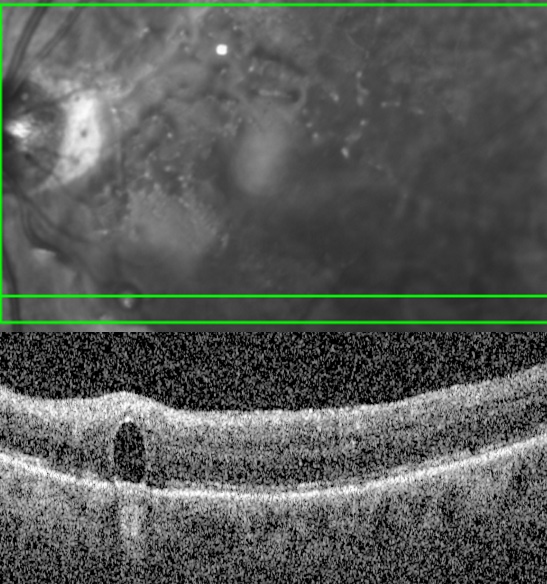
Retina phototoxicity
Occurs due to the operating microscope or the endoilluminator. Higher risk when the light source is higher intensity, higher duration, and in closer proximity to the retina. Photosensitizing agents such as history of systemic tetracycline use or use of indocyanine green dye during surgery increase the risk.
Risk mitigation
- Turn the operating scope off when not actively being use
- Turn the operating scope off and remove the endoilluminator when allowing instilled indocyanine green dye to stain the macula. Remove the dye promptly.
- Limit intensity, duration, and proximity (esp when working in the macula) of light sources
Clinical features: whitish macular lesion which over time evolves to a pigmented lesion with ensuing scar. Patients may note a scotoma
Retinal phototoxicity
Photos by Kyle Kovacs, MD
Management: none, prevention is key
Exogenous postoperative endophthalmitis
Rare (0.03 – 0.05% risk after vitrectomy)
Typically manifests between 3 – 10 days postoperatively
Clinical features: pain, decreased vision, conjunctival injection, anterior chamber cell, hypopyon, vitritis
Evaluate for wound leaks, vitreous incarceration, suture abscess, exposed sutures for secondary intraocular lens placement
Management
- Tap of vitreous and aqueous for gram stain and culture, followed by intravitreal injection of antimicrobials. Intravitreal dexamethasone may be considered at the same time (if no suspicion for fungal etiology) or subsequently
- Pars plana vitrectomy, especially in severe cases, cases with fulminant . organisms, or cases not responding sufficiently to medical managemenet as above
Sympathetic ophthalmia
Rare (〈0.01% risk)
Risk factors in addition to surgery: history of open globe injury
Clinical findings: bilateral uveitis, mutton fat keratic precipitates, anterior chamber cell, vitreous cell,serous retinal detachments, Dalen Fuchs nodules, choroiditis, may have optic disc edema
Management: steroids and/or immunosuppressant therapies
Other postoperative complications
Retinal fold

Retinal fold after retinal detachment repair
Photo by Anton Orlin, MD
A fold in the retina after detachment repair, especially with use of a gas tamponade – through vitrectomy >> buckle or pneumatic
Symptoms include decreased vision, metamorphopsia – especially for macular folds
Risk factors
- Use of a gas tamponade, incomplete drainage of subretinal fluid, bullous superior retinal detachments, slippage associated with giant retinal detachment repair, acute (vs. chronic) detachments, large circumferential buckles, poor compliance with positioning
Risk mitigation
- Try to drain subretinal fluid as completely as possible.
- Consider temporal side down positioning in the acute postoperative period before initiating face down positioning. This is especially helpful in cases where there may be a delay in face-down positioning in the OR or in the PACU (e.g. general anesthesia cases)
Management
- Observation
- Always observe asymptomatic folds
- Symptomatic folds may also be observed as some folds may resolve spontaneously. Folds should be monitored every few weeks and if improving, may be monitored. This is especially the case for “pseudo-folds” – folds that are not full thickness, thus involving either only the inner or only the outer retina.
- Surgery
- Indicated for symptomatic folds, especially if no improvement upon close monitoring
- A localized retinal detachment involving the area of the fold is created (avoiding the fovea if possible) by infusion of BSS using a 41g canula to release the fold. The retina is reattached with PFO. A peripheral retinotomy is made to enable subretinal fluid drainage during fluid-air exchange. The patient is left with a tamponade and face down positioning.
Repair of a retinal fold after retinal detachment surgery
Video by Anton Orlin, MD
SCLERAL BUCKLE -- INTRAOPERATIVE CHALLENGES AND COMPLICATIONS
Back to topComplications during exposure
Damage to rectus muscles
Risk mitigation: careful dissections (especially in re-operations with scarring) at 12, 3, 6, and 9 o’clock
Damage to the levator
Risk mitigation: avoid extensive dissection along the superior rectus
Loss of extraocular muscle
In cases where muscles are removed during scleral buckling and lost.
Attempt to retrieve and reattach the muscle. If unable, continue with buckle procedure as planned and refer to strabismus surgeon for future retrieval and repair of the muscle.
Complications from suturing or belt loops
Damage to the vortex veins (risk of choroidal hemorrhage)
- Risk mitigation: careful examination of the sclera for areas of thinning and vortex veins and avoidance of suture passes in these areas
Scleral perforation
- Risk factors: thin sclera, myopic eyes, technique
- Risk mitigation
- Careful examination of the sclera for areas of thinning and avoidance of suture passes in these areas
- Suture pass technique to ensure appropriate engagement and depth of suture passes. For encircling bands suture passes need not be long nor deep in particular. Elements whose heights are achieved by imbrication require more firmly but appropriately placed suture passes.
- Use of spatulated needle (which is flatted in antero-posterior dimention to facilitate needle pass within the intrascleral plane)
- Care when withdrawing the needle to ensure the needle is not lifted so as to allow the heel of the needle to tilt backwards and perforate the eye
- Signs
- Blood or pigment at the site of scleral suture passes or egress of subretinal fluid or vitreous at the suture site
- Management
- Evaluation of the fundus with indirect ophthalmoscopy to determine the depth of perforation.
- If the pass was limited to the choroid or subretinal space, the retina will appear unaffected, subretinal fluid egress will cease, and no additional maneuvers are necessary.
- If the retina was affected a break, white fleck, or retinal hemorrhage may be noted on indirect ophthalmoscopy and fluid may continue to egress from the site.
- If significant bleeding is noted, pressure should be applied to the perforation site external to attempt tamponade. Suture of the perforation site may be considered to facilitate elevation of IOP. The eye should be positioned to avoid gravitational flow of subretinal hemorrhage to the fovea, especially in macula off detachment cases. Smaller hemorrhages may be observed. Gas (0.3cc pure air or gas) may be instilled to facilitate pneumatic displacement from the macula.
- If massive choroidal or subretinal hemorrhage is noted, vitrectomy with internal drainage of subretinal blood may be considered.
- The retinal break needs to be treated and the buckle position adjusted accordingly (by adjusting the position of the buckle posteriorly to cover the additional break or adding an additional element)
- With belt loops, perforation through the sclera, choroid, and if relevant, retina, can result in an open globe. If an open globe in noted, 8-0 nylon sutures should be used to close the rupture site. Associated suprachoroidal hemorrhage and retinal breaks should be managed as above.
Complications from subretinal fluid drainage
Risk mitigation
- Drain in area of maximal subretinal fluid
- Avoid drainage sites near major choroidal vessels or thin sclera
Intraocular and choroidal hemorrhage
- Minor subretinal hemorrhage or bleeding from the choroidal drainage site: observe
- More significant bleeding
- Subretinal hemorrhage
- Noted on indirect ophthalmoscopy
- Management
- Position the eye to prevent migration of subretinal blood c components under the fovea
- Compression/pressure on the globe to elevate IOP to achieve tamponade
- Air or gas (0.3cc pure air or gas) may be instilled to facilitate pneumatic displacement from the macula.
- If massive subretinal blood noted, consider vitrectomy with drainage of subfoveal hemorrhage
- Choroidal hemorrhage
- Noted as dark fluid egressing from the drainage site
- Management
- Close the drainage site
- Compression/pressure on the globe to elevate IOP to achieve tamponade
Retinal perforation
- Risk factors: Excessive needle entry or shallow subretinal fluid
- Signs: white/yellow fleck, hemorrhage, or break noted on indirect ophthalmoscopy
- Risk mitigation: drain in the area of most subretinal fluid, careful drainage technique
- Apply cryotherapy or laser (if retina flat) to the break and ensure buckle covers the break (adjust position or add element if needed. This can be avoided by always draining in the bed of the buckle)
Retinal incarceration
- Risk factors: elevated pre-operative intraocular pressure, large drainage site
- Signs: sudden stop of egress of subretinal fluid; fundus indirect ophthalmoscopy revealing folds and dimpling of the retina emanating from the incarceration site
- Support the site with the buckle
- May require vitrectomy with retinectomy
Management:
Complications from cryotherapy
Scleral rupture
- Due to movement of the probe while still frozen to the eye wall, resulting in rupture of choroidal vessels (and choroidal or subretinal hemorrhage) or scleral avulsion and rupture
- Risk mitigation: avoid moving or removing the cryotherapy probe after application of cryotherapy until sufficient time for the probe to thaw and release attachment to the sclera
- Management
- Small scleral avulsion/rupture sites can be sutured as with open globe repair
- Larger defects may require donor sclera or a pericardial patch graft
- Shafting of the probe causing inadvertent posterior/macular cryotherapy
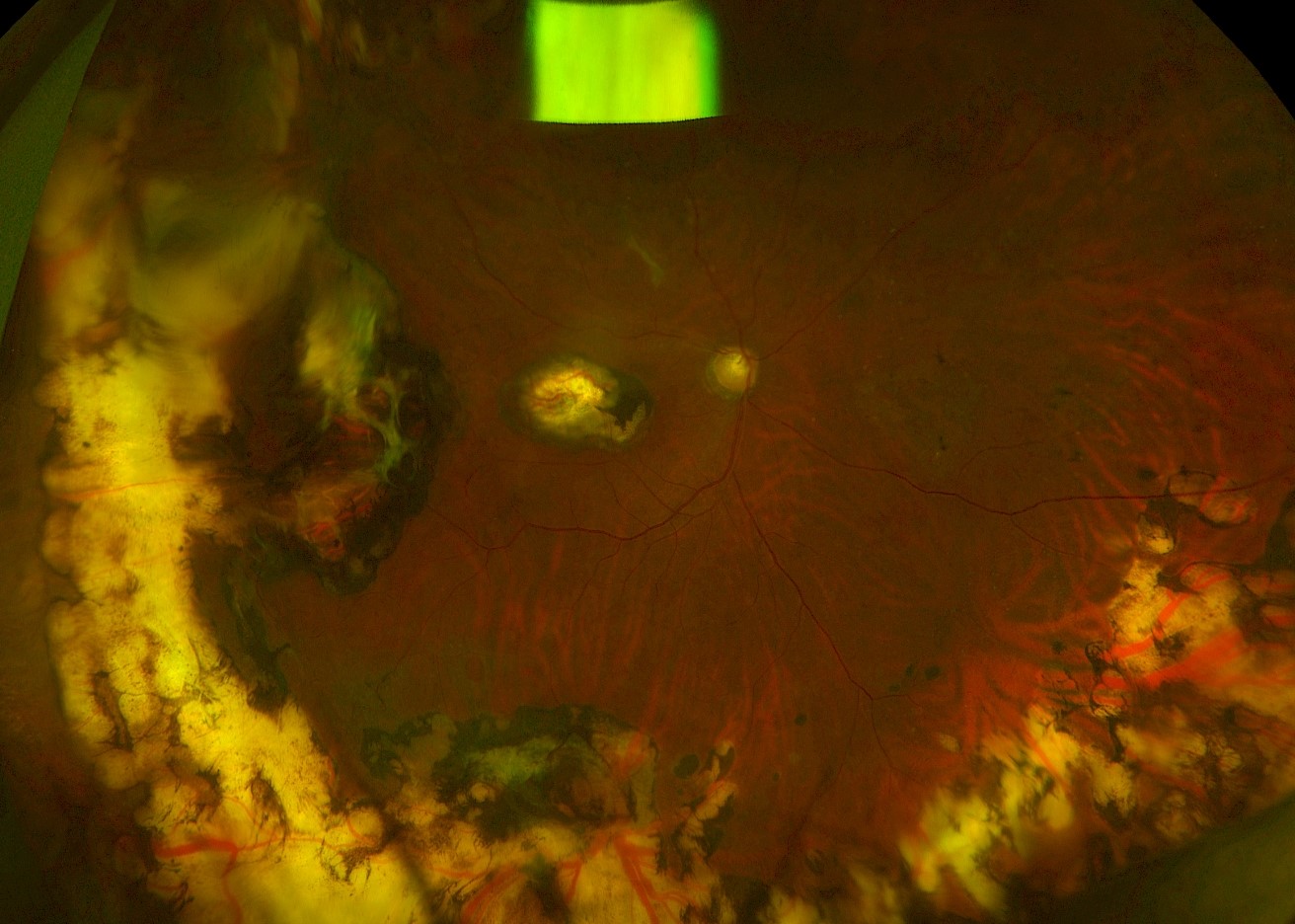
Inadvertant posterior cryotherapy due to shafting of the probe
SCLERAL BUCKLE -- POSTOPERATIVE COMPLICATIONS
Back to topFailure to re-attach
Causes
- Incorrect buckle position
- Missed or new (and therefore unsupported) retinal breaks
- Proliferative vitreoretinopathy
Of note: always consider that if subretinal fluid is not drained, and especially if chronic in duration prior to repair, the subretinal fluid may take months to resorb. Factors supporting persistent subretinal fluid rather than failure to reattach include (1) all breaks are well supported by the buckle, (2) no new breaks, (3) fluid does not increase with time (may be very slow to resorb).
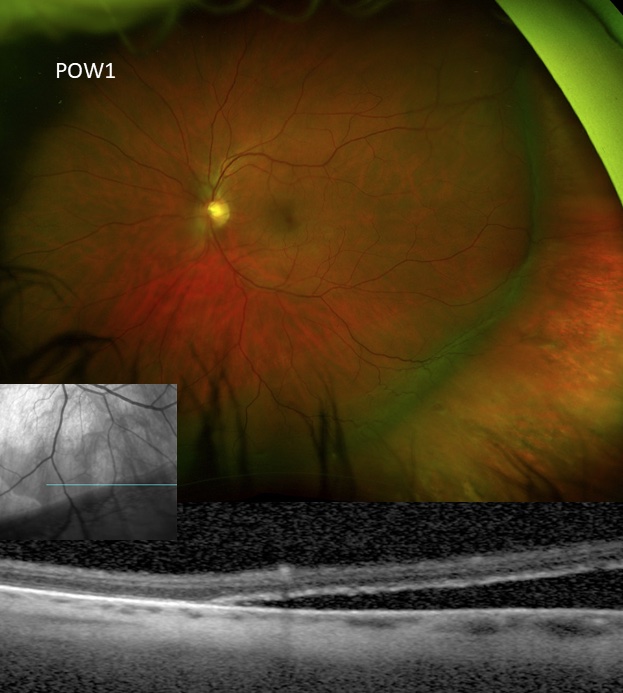
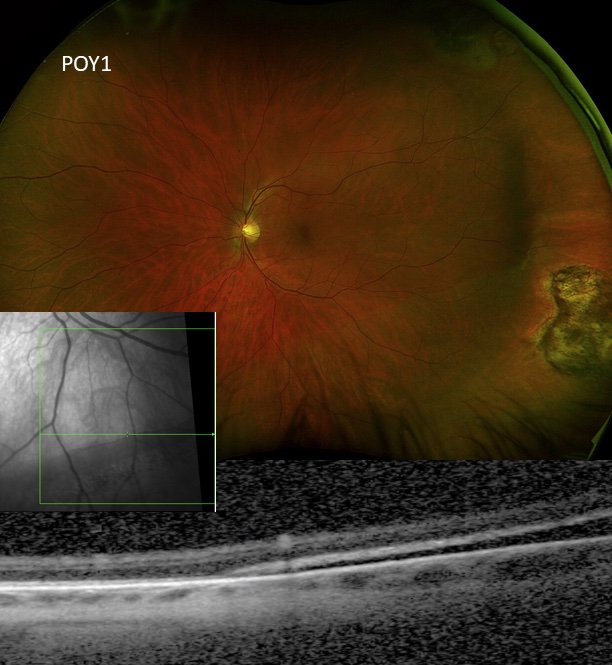
Slow resorption of persistent subretinal fluid due to non-drainage buckle surgery
Photo by Mark Breazzano, MD
Elevated intraocular pressure
Open angle
- Due to inflammation, inflammatory cells/hemorrhage/etc obstructing the trabecular meshwork postoperatively, steroid response, gas overfill, and/or reduction in the intraocular volume (especially with large or high buckles)
- Topical or systemic medical management usually sufficient
- Generally normalizes after the immediate post-operative pressure
Angle closure
- With pupillary block or without pupillary block
- More common with large or high circumferential or encircling elements
- Due to anatomical changes from the buckle, including congestion of the vortex vein system resulting in choroidal detachment (serous; rarely hemorrhagic); gas; anterior displacement of the crystalline lens; aqueous misdirection
- Management: step-wise until IOP control achieved:
- Topical or systemic IOP-lowering medication
- If the above fails and due to pupillary block component, laser peripheral iridotomy
- If the above fails and due to gas, face down positioning or removal of some gas
- If choroidal detachments:
- Topical and systemic steroids
- Atropine
- If persistent and severe IOP elevation despite the regimen above (or retinal apposition), choroidal drainage -- with anterior chamber reformation if needed
- Techniques for drainage
- Cut down with blade
Choroidal drainage technique: scleral cut down
Video by Thomas Lazzarini MD + Harry Flynn MD
- Use of the cannula (valve removed)
- If the above are insufficient, consider buckle revision (loosening, cutting, or removing)
Anterior segment ischemia
- Pain, IOP elevation, corneal edema, anterior chamber inflammation, shallow anterior chamber, mid-dilated pupil, and later findings such as iris atrophy, posterior synechiae, cataract
- Topical (and systemic, if needed) steroids and IOP-lowering therapy
- If the above is insufficient, cutting or loosening of the encircling band
Choroidal effusion
Due to anatomical changes from the buckle which lead to congestion of the vortex veins
IOP may be elevated
Management:
- Topical and systemic steroids
- Atropine
- Topical or systemic IOP-lowering medications as needed
- If persistent and severe IOP elevation despite the regimen above or retinal apposition, choroidal drainage -- with anterior chamber reformation if needed
- Techniques for drainage
- Cut down with blade
Choroidal drainage technique: scleral cut down
Video by Thomas Lazzarini MD + Harry Flynn MD
- Use of the cannula (valve removed)
Suprachoroidal hemorrhage
Risk factors: Older patients, hypertension or tachycardia, anticoagulation, history of glaucoma (especially with pre-operative IOP elevation), high myopia, aphakia, pseudophakia, coughing/bucking, perforation during suture passes, external drainage of subretinal fluid
Management
- Small choroidal hemorrhages often resolve and can be managed medically with topical and systemic steroids, atropine, and medications for IOP lowering as needed. Large hemorrhages or increasing hemorrhages resulting in kissing choroidals or other complications may require subsequent drainage, ideally 10 – 20 days postoperatively to allow time for the eye to quiet down / stabilize and for clot lysis
- Techniques for drainage
- Cut down with blade
Choroidal drainage technique: scleral cut down
Video by Thomas Lazzarini MD + Harry Flynn MD
- Use of the cannula (valve removed)
- • Use of PFO for internal pressure to facilitate drainage
Exudative retinal detachment
Usually starts to occur several days postoperatively, color more turbid than rhegmatogenous fluid, usually inferior; fluid shifts based on head position
Risk factors include longer surgery, large amounts of crytherapy application
Management: resolves over time; consider topical and systemic steroids.
Buckle extrusion and infection
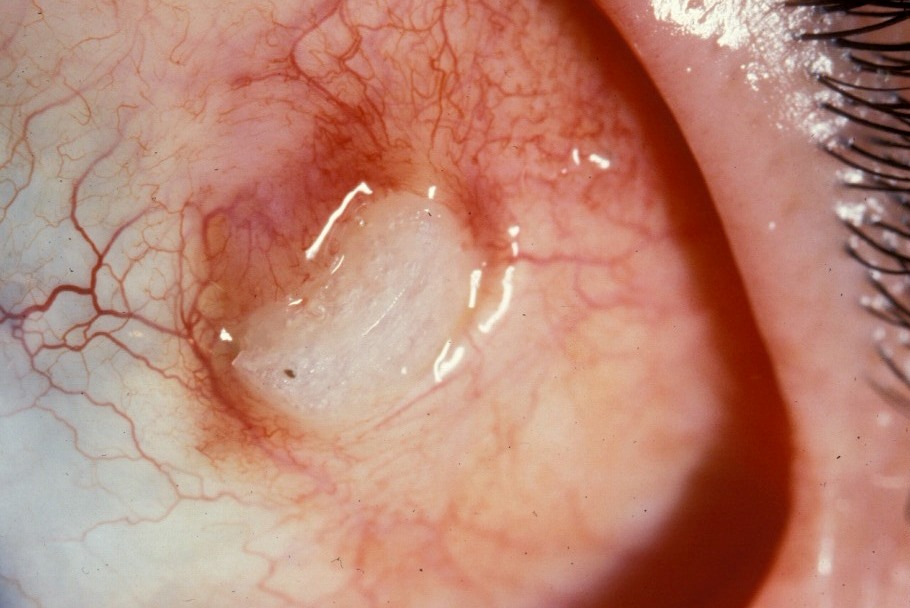
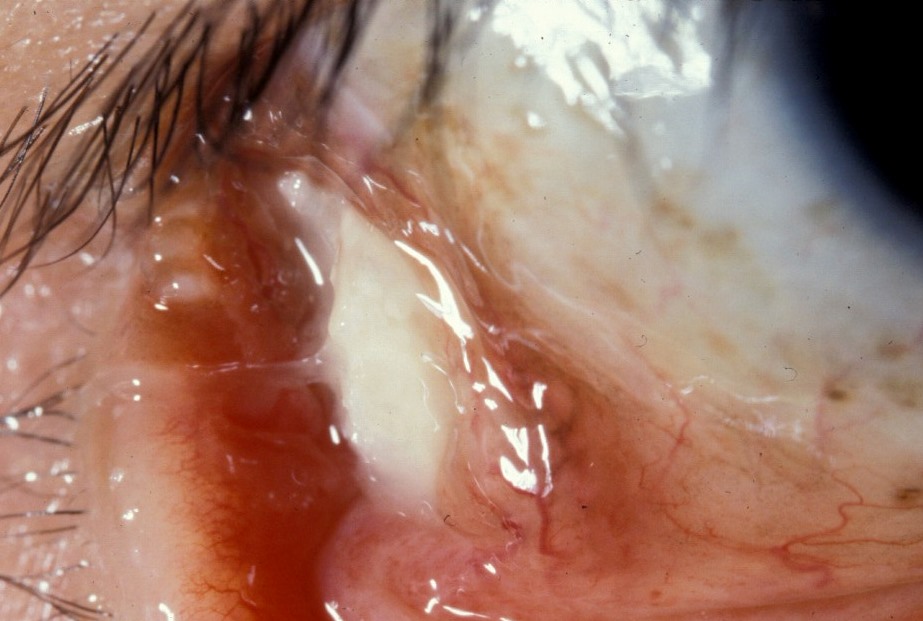
Scleral buckle extrusion
Photo by Donald J. D'Amico, MD
Risk factors for extrusion: large elements, silicone sponges, inadequate suture bites allowing anterior migration
Topical antibiotics until scheduled buckle removal or revision (covering with donor sclera or pericardium grafts). There are reports of long-term observation with ongoing topical antibiotic therapy.
If secondary infection suspected (mucupurulent discharge, significant pain, etc), then systemic antibiotics may also be considered and the buckle should be removed promptly
Eye culture should be performed and the removed buckle components sent to microbiology to guide antibacterial therapy
Irrigation of the operative area with iodine +/- antibiotics during buckle removal surgery should be considered, especially with an infected buckle
Risk of re-detachment after buckle removal is low but patients should be counseled
Buckle intrusion
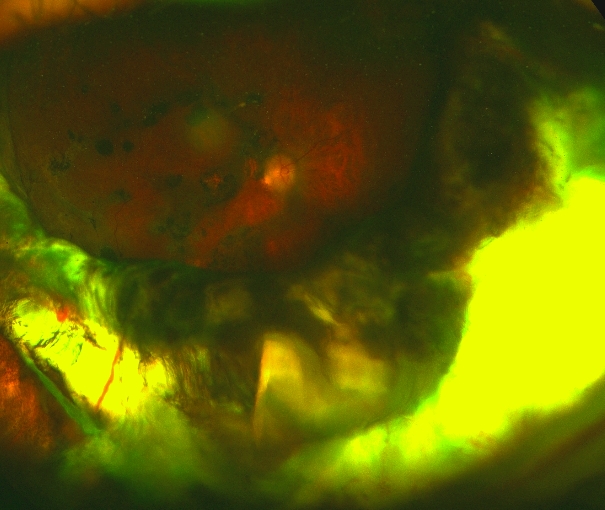
Scleral buckle intrusion
Photo by Szilard Kiss, MD
Uncommon
Risk factors: high myopia, large or high buckles, extensive cryotherapy
Signs: visible intrusion on examination/ultrasound, recurrent detachment, recurrent vitreous hemorrhage, hypotony, endophthalmitis risk
Intervention
- Individualized approach
- Indicated if: recurrent detachment, vitreous hemorrhage, danger to integrity of the eye (leaking, hypotony, etc)
- Cutting an encircling component may be enough to relieve traction, depending on the buckle
- Removal of the buckle may be necessary. Scleral patch graft should be available to repair the scleral defect.
